The Chemistry Behind Pystene Descaling Products
Citric and lactic acids work effectively as descaling agents because they are both organic acids that can dissolve mineral deposits through chemical reactions.
Scale buildup typically consists of calcium carbonate (limescale), calcium sulfate, or other mineral deposits that form when hard water evaporates. These deposits are alkaline in nature, and acids can effectively break them down.
Citric Acid
Citric Acid
Citric Acid

Effectiveness of Citric Acid
Citric acid is particularly effective because:
- It forms complexes with metal ions (chelation), which helps pull minerals away from surfaces
- It has multiple acid groups that can react with scale deposits
- It's strong enough to dissolve scale but mild enough not to damage many surfaces
Lactic Acid
Citric Acid
Citric Acid
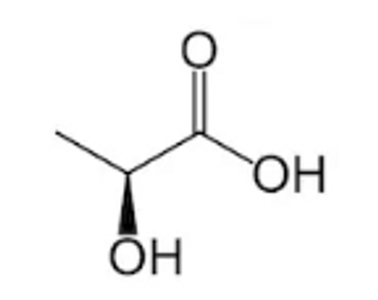
Effectiveness of Lactic Acid
Lactic acid is particularly effective because:
- It has good penetrating properties that help it work into thick scale deposits
- It tends to be less corrosive to metals than some stronger acids
- It's effective at removing both mineral deposits and soap scum
Ingredients in order of concentration

Purified Water
Citric Acid
Lactic Acid

Citric Acid
Lactic Acid
Calcium Lactate (Byproduct of Lactic Acid Production)
Links to Product Safety Data Sheets
Copyright © 2025 Prystene - All Rights Reserved.
Powered by Science
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.
